Transcatheter Aortic Valve Replacement (TAVR) or Transcatheter Aortic Valve Implantation (TAVI) is a procedure for replacement of the aortic valve of the heart through the blood vessels. This minimally invasive procedure is done for a condition known as “aortic stenosis” in which the heart valve that propels blood from the heart to the rest of the body becomes narrowed. The Centers for Medicare & Medicaid Services (CMS) covers TAVR for the treatment of symptomatic aortic valve stenosis through Coverage with Evidence Development (CED). CMS recently updated its national coverage policy for Transcatheter Aortic Valve Replacement. Outsourcing medical billing as well as coding is a great option for cardiologists to document TAVR as well as other treatment options for aortic stenosis and get reimbursed on-time.
Based on the latest update, CMS will continue to cover TAVR under coverage with evidence development (CED) when furnished according to an FDA-approved indication. The update is also applicable for the coverage criteria for hospitals and physicians to begin or maintain a TAVR program.
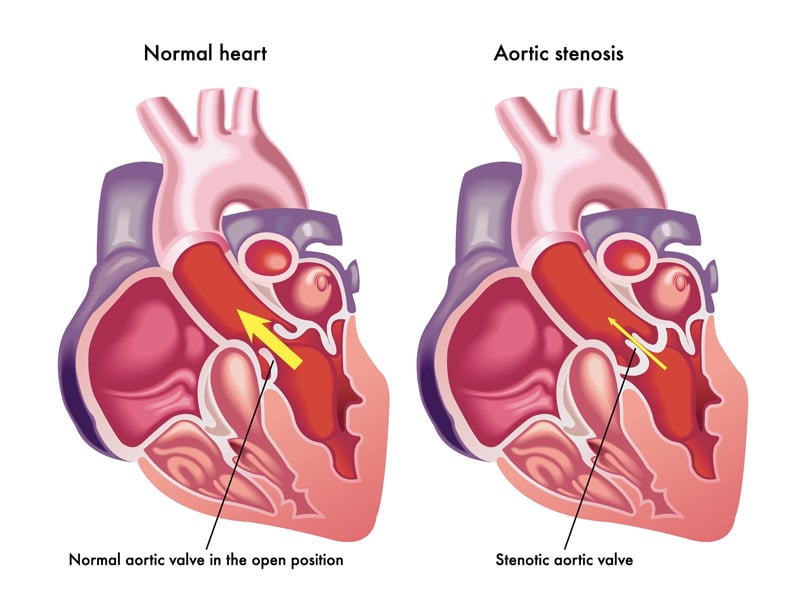
Aortic valve stenosis – or aortic stenosis – occurs when the heart’s aortic valve narrows and can lead to chest pain, fainting, fatigue, leg swelling and shortness of breath and even heart failure and sudden cardiac death. TAVR is often recommended for people who are considered at intermediate or high risk of complications from surgical aortic valve replacement and for those who can’t undergo open-heart surgery.
In 2012, CMS had released a National Coverage Decision (NCD) which provides details of the qualifications of the physicians who perform TAVR and the patients who benefit from the procedure. CMS has now updated NCD for above 150 hospitals performing TAVR.
TAVR NCD – Updated Requirements
If adopting TAVR program for the first time or experienced in the technique, it is critical for facility and physicians to fulfill certain requirements.
- The facility should use FDA-approved supplies and instruments to perform the procedure
- The patient undergoing the procedure must be under the care of a multi-disciplinary heart team preoperatively and postoperatively
- IVR cardiologists and cardiac surgeons must jointly participate in the intraoperative technical aspects of TAVR
- Hospitals must perform at least 50 TAVRs and more than 300 percutaneous coronary interventions per year
Hospitals must participate in a prospect, nation, audited registry that follows TAVR patients for at least a year and monitors – Stroke, All-cause mortality, Transient Ischemic Attacks (TIAs), Major vascular events, Acute kidney injury, Repeat aortic valve procedures, New permanent pacemaker implantation, and Quality of Life (QoL).
It is noted that TAVR must be furnished in a hospital with the appropriate infrastructure that includes but is not limited to – on-site heart valve surgery and interventional cardiology programs and post-procedure intensive care facility with personnel experienced in managing patients who have undergone open-heart valve procedures.
Facilities may also perform TAVRs not expressly listed as an FDA-approved indication when performed within a clinical study if it fulfills standard and added research protocol. However, this clinical study must adhere to certain standards of scientific integrity and relevance to the Medicare population such as – the rationale for the study should be well supported by available scientific and medical evidence, the study needs to be sponsored by an organization or individual capable of completing it successfully, and all aspects of the research study are to be conducted according to appropriate standards of scientific integrity.
TAVR Medical Coding
Be sure to correctly note the families of arteries through which the catheters are routed. Professional medical coding companies will provide the services of experienced medical coders who are up to date with the changing coding regulations and guidelines for the cardiology specialty.
TAVR cardiovascular access and delivery procedures are reported with
CPT Codes
- 33361 Transcatheter aortic valve replacement (TAVR/TAVI) with prosthetic valve; percutaneous femoral artery approach
- 33362 —————; open femoral artery approach
- 33363 —————; open axillary artery approach
- 33364 —————; open iliac artery approach
- 33365 —————; transaortic approach (e.g., median sternotomy, mediastinotomy)
- 33366 —————; transapical exposure
Add-on codes for bypass
- 33367 Transcatheter aortic valve replacement (TAVR/TAVI) with prosthetic valve; cardiopulmonary bypass support with percutaneous peripheral arterial and venous cannulation (e.g., femoral vessels) (List separately in addition to code for primary procedure)
- 33368 —————; cardiopulmonary bypass support with open peripheral arterial and venous cannulation (e.g., femoral, iliac, axillary vessels) (List separately in addition to code for primary procedure)
- 33369 —————; cardiopulmonary bypass support with central arterial and venous cannulation (e.g., aorta, right atrium, pulmonary artery) (List separately in addition to code for primary procedure)
Aortic Valve Stenosis diagnosis can be documented with ICD-10 codes, which we have discussed in our blog. For adequate documentation of the procedure, the coders must be up-to-date with the changing coding guidelines.